Academic Emergency Medicine Publishes Results from EPISODE-PS-COVID Revealing Harmony Can Reliably Identify LVO Stroke
MindRhythm’s Device, Harmony, is a Feasible Tool for Use in the Prehospital Setting
CUPERTINO, CALIFORNIA, UNITED STATES, April 22, 2024 /EINPresswire.com/ -- MindRhythm Incorporated, a neurodiagnostic technology company based in Cupertino, California, today announces that results from the pilot phase of its 3 phase clinical study EPISODE have been published. The article entitled: "Headpulse measurement can reliably identify large-vessel occlusion stroke in prehospital suspected stroke patients: Results from the EPISODE-PS-COVID study" was published by Academic Emergency Medicine. While pilot EPISODE data has been presented in various academic society forums, this is the first official publication of EPISODE results. The journal is the monthly publication for the Society for Academic Emergency Medicine (SAEM). It is one of the largest peer reviewed global journals in emergent care. It supports the objectives of SAEM to promote scientific discovery and innovation for clinicians, researchers, and educators.The family of EPISODE studies (hEadpulse for Ischemic StrOke Detection Prehospital) is the largest evaluation of a prehospital stroke device with 8 geographies, 17 hospitals and 14 EMS groups participating in this research. Primary Investigator Dr. James Paxton, MD of Wayne State Medical in Detroit, Michigan leads the research with the support of 18 subinvestigators throughout the country. Dr. Paxton submitted the manuscript to the journal highlighting the results of pilot study EPISODE-PS-COVID on behalf of the entire research team. The abstract for this publication was awarded “Best Overall Abstract” in October of 2023 by the American College of Emergency Physicians Research Committee. This award was granted to Dr. Paxton out of approximately 700 abstracts signifying the importance of this research.
Results from the EPISODE-PS-COVID, pilot phase reveal that MindRhythm’s cranial accelerometry based device, named Harmony, is a feasible tool for use in the EMS-prehospital setting, and can reliably identify large vessel occlusion (LVO) stroke. Use of the device algorithm incorporating subjective data from a commonly used stroke scale: Los Angeles Motor Scale (LAMS), and Harmony’s cranial accelerometry data resulted in “significantly higher sensitivity without reduced specificity when compared to the use of LAMS alone,” writes the author Dr. James Paxton, MD. The goal of effectively identifying LVO stroke prehospital, is to improve the triage decision. This knowledge ensures a critical patient arrives at the hospital equipped with the proper treatment tools, which is the difference between life, death, and disability.
Harmony is a diagnostic tool that is designed to identify stroke type-primarily a large vessel occlusion (LVO) in a prehospital setting. Data suggests that currently 70-75% of LVO patients are triaged to the wrong hospital. The standard prehospital method of identifying a stroke type is a subjective assessment of symptoms plugged into various scales and scores. Once a stroke is suspected, regardless of type, standard practice is to transport the stroke patient to the nearest hospital which in most cases is a hospital that is not thrombectomy equipped to surgically remove the clot from a LVO stroke patient’s brain. While any stroke is debilitating, a LVO is the most debilitating and often fatal type of stroke. Patient success relies on immediate removal of the clot blocking the flow of blood to the key areas of the brain. MindRhythm’s technology is designed to improve outcomes for patients by ensuring that at the start of care, in the prehospital setting, the best triage to treatment decision for the patient is made.
MindRhythm was granted a Breakthrough Device Designation by the FDA as a result of the data from EPISODE-PS-COVID. The combined data sets from the pilot and pivotal phases of the ultimate EPISODE study will be used for MindRhythm’s FDA submission.
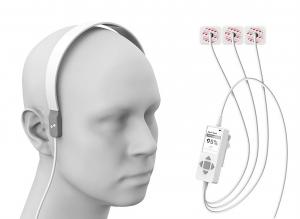
About the Harmony Headset
Harmony is a novel, noninvasive, medical device designed to aid in the rapid identification of Large Vessel Occlusion (LVO) strokes, the most debilitating of all stroke types. Harmony monitors a newly discovered physiology MindRhythm calls the “HeadPulse”. HeadPulse is measured by applying a highly sophisticated sensor to the patient’s head, measuring minute pulsations produced by each heartbeat. The HeadPulse changes dramatically during LVO strokes, which is read by Harmony. Coupling the Harmony results with a simple clinical examination of stroke, the device can discriminate LVO strokes among stroke patients in a matter of seconds.
About MindRhythm
MindRhythm is a medical technology company focused on preventing neurological injury. Founded by world-renowned scientific experts with significant commercialization success, MindRhythm’s monitoring technologies provide real-time visibility to life-threatening situations at home, prehospital, in the operating room, and on the field. MindRhythm’s technologies allow clinicians to intervene, optimize, and manage care to prevent brain damage. Collaborating with the healthcare community, MindRhythm looks to apply a systematic approach to reducing time to treatment in strokes and monitor neurological health during recovery from injury. Together, let’s save lives and improve the quality of life: https://mindrhythm.com
Beth Johnson
MindRhythm Incorporated
+1 860-836-8630
email us here
Visit us on social media:
Twitter
LinkedIn
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.